Bivalent vaccine
Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains. The updated version of the Pfizer-BioNTech.

Moderna Says Its New Bivalent Vaccine Shows Promise Shots Health News Npr
Original COVID-19 vaccines which have been authorized and administered to millions of Americans since 2020 are now referred to as monovalent According to the FDA.

. The bivalent vaccine targets the original omicron variant. A bivalent vaccine elicits an immune response against two different antigens. This can means two different viruses or two variations of one virus.
The bivalent booster is the most recent version of the COVID-19 vaccine. New bivalent COVID vaccine may not be more protective but its still recommended. Vaccination with the bivalent Covid-19 mRNA booster vaccines has began.
BA4 and BA5 share many similarities to the original variant but also appear to have mutations that make them more. An updated version of Modernas Covid-19 booster shot appears to work against the fast-spreading omicron subvariants BA4 and BA5 the company said in a news release. Health Canada has approved Pfizers new bivalent COVID-19 vaccine which targets the virus strains now most common in Canada.
The safety of a single booster dose of the Moderna COVID-19 Vaccine Bivalent for individuals 18 years of age and older is supported by safety data from a clinical study which. A booster program a. The Ministry of Health MOH will be bringing forward the administration of the bivalent ModernaSpikevax vaccine from 17 October 2022 which was previously announced.
Health Canada has authorized another Moderna bivalent COVID-19 booster vaccine for those 18 years and older to tackle the Omicron BA4 and BA5 subvariants of COVID-19 two. Isaac Bogoch expects we could be running three simultaneous vaccine programs. The results showed that a booster of the bivalent vaccine triggers a strong immune response against both the original Wuhan strain and omicron BA1.
They compared virus neutralization by sera collected from individuals vaccinated with three doses of the original monovalent vaccines and a fourth dose of the bivalent vaccine. Households to get antigen rapid test kits. These bivalent vaccines help to create a broader immune response and improve the strength and duration of protection against circulating variants.
It contains both the original vaccine strain of the virus and a strain derived from the BA5 omicron variant. If bivalent shots are ready for fall infectious disease expert Dr. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been.
Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully provided immunogenicity a boost to your immunity and elicited. The new bivalent COVID-19 vaccine includes mRNA from the original strain of SARS-CoV-2. The current COVID-19 vaccines.
The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing. Kaiser nurse Marilyn Antonio left gives a Moderna booster shot to Ted Naifeh right from San. The bivalent is designed to offer better protection against the more recent Omicron variants.
This helps to create a broader immune. Bivalent Covid-19 booster jabs extended to those aged 18 to 49 from Nov 7. A bivalent vaccine however contains mRNA components from two strains of virus.
The Moderna bivalent vaccine is only registered for use as a booster vaccine and contains 50mcg of mRNA comprising equal quantities encoding the spike protein from the.

Ccchd Pausing Covid 19 Booster Shots Ahead Of Authorization For Bivalent Vaccine Prevent Promote Protect
/cloudfront-us-east-2.images.arcpublishing.com/reuters/GT64FVRGFVOPVNQC5SD3FFJ56Y.jpg)
U S Sets 1 74 Billion Deal With Moderna For Updated Covid Vaccine Reuters

Fda Recommends Omicron Bivalent Covid 19 Boosters For Fall Infectious Disease Special Edition

Health Canada Approves Moderna S Covid 19 Vaccine For Omicron Ba 4 Ba 5 Variants Cbc News

Moderna Says Its New Vaccine Booster Shows Superior Response To Omicron Npr
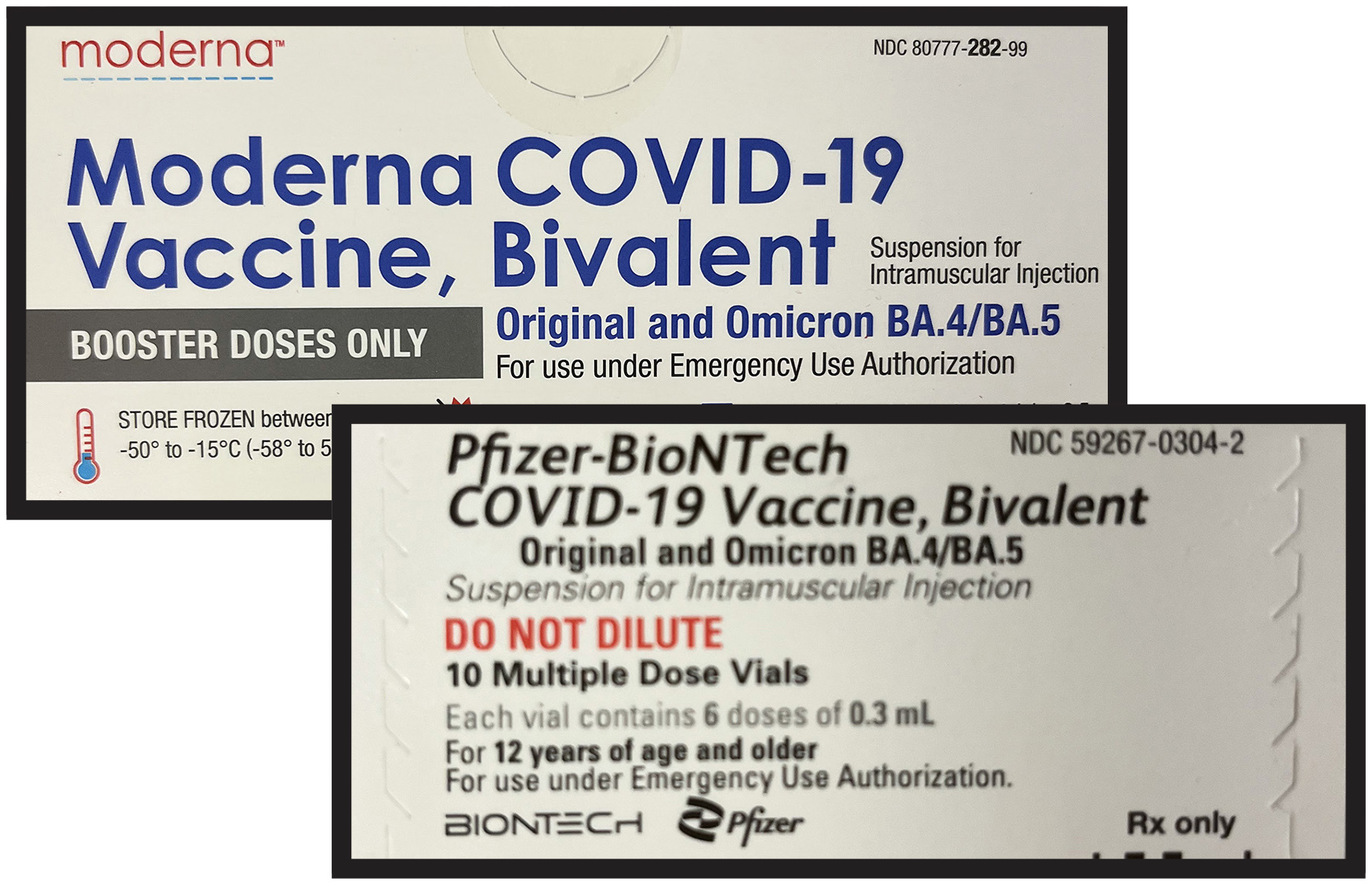
Wayne Memorial Opens Appointments For Bivalent Covid Booster Clinics Wayne Memorial Hospital

Northeast Ohio Counties Roll Out Plans For New Covid Bivalent Vaccine Clinics Wksu
Ask The Doctors New Bivalent Boosters Long Covid And When To Get Your Flu Shot Radio Boston
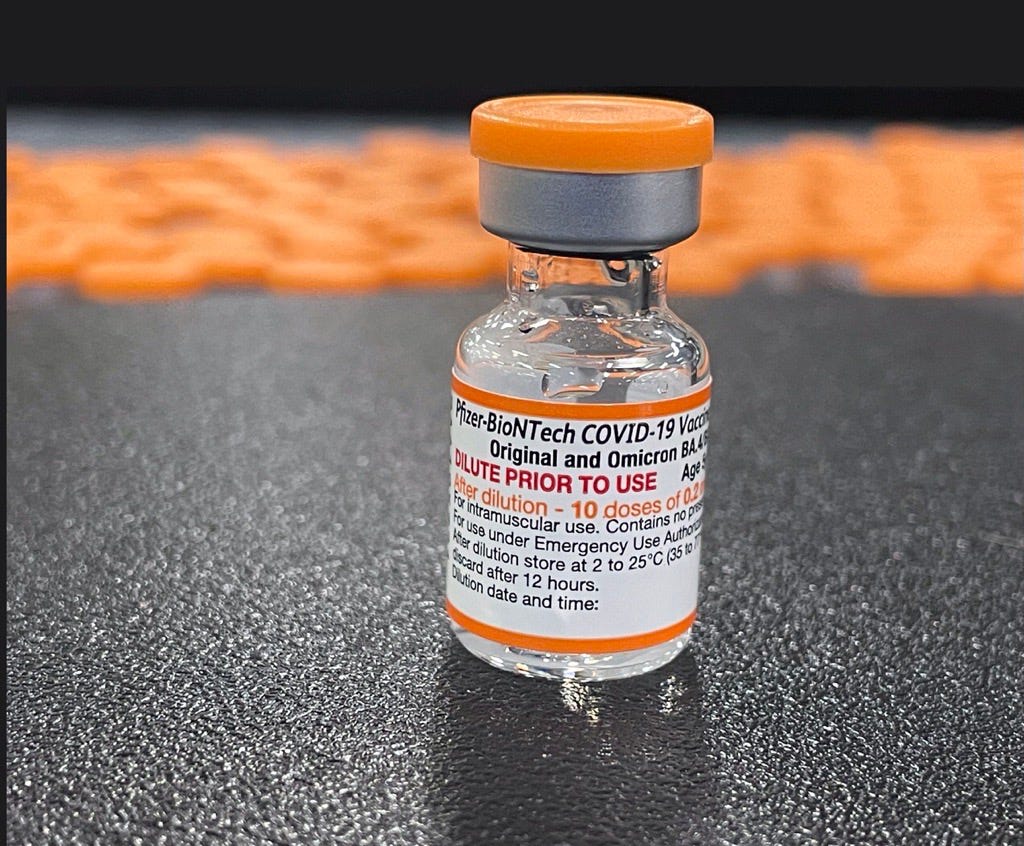
Covid Bivalent Booster For Kids Under 12 Authorized By Fda

Moderna S Bivalent Booster Becomes First Authorized Omicron Vaccine
Pfizer Asks Fda To Authorize Bivalent Covid 19 Vaccine For Children Age 5 11 Aha News

Jcdhe To Begin Offering Bivalent Covid 19 Boosters Johnson County Kansas

Covid 19 Vaccination Information Arkansas Department Of Health
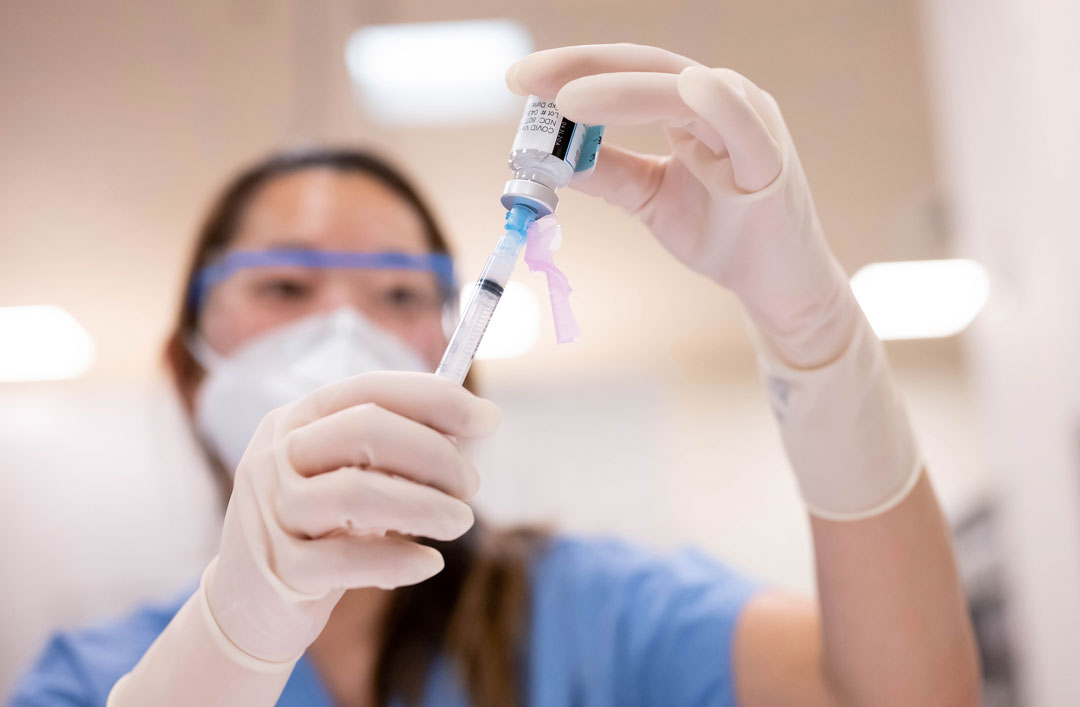
Omicron S Evolution And The New Bivalent Covid 19 Booster Uc San Francisco

British Regulator 1st To Authorize Moderna S Updated Covid Booster Cbc News
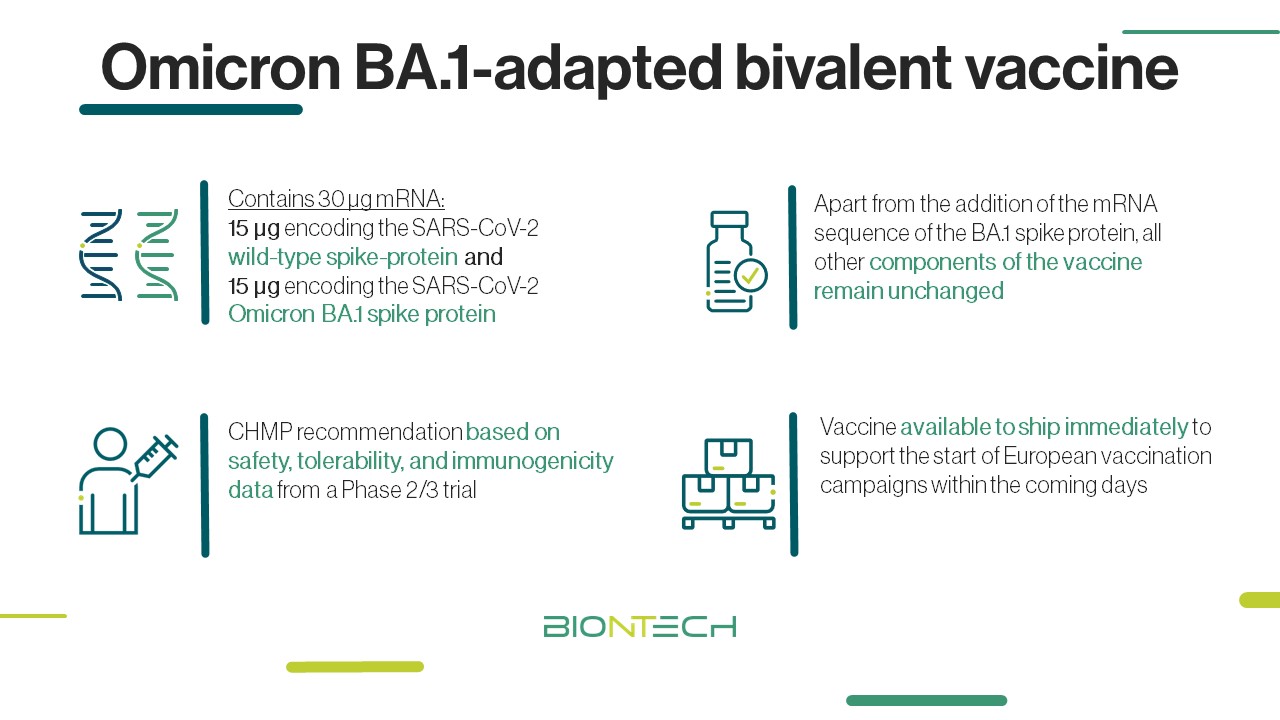
Biontech Se On Twitter Omicron Ba 1 Adapted Bivalent Vaccine Update Our Ba 1 Bivalent Covid 19 Vaccine Was Recommended For Conditional Marketing Authorization By Ema News Committee For Medicinal Products For Human Use As A Booster

A Bivalent Omicron Containing Booster Vaccine Against Covid 19 Nejm

